Medical Writing Market to Reach USD 10.26 Billion by 2032, Boosted by Regulatory and Clinical Demands | Coherent Market Insights
Rising demand for regulatory documentation, growth in clinical trials, increased outsourcing, expanding healthcare sectors, and the need for clear scientific communication drive the medical writing market
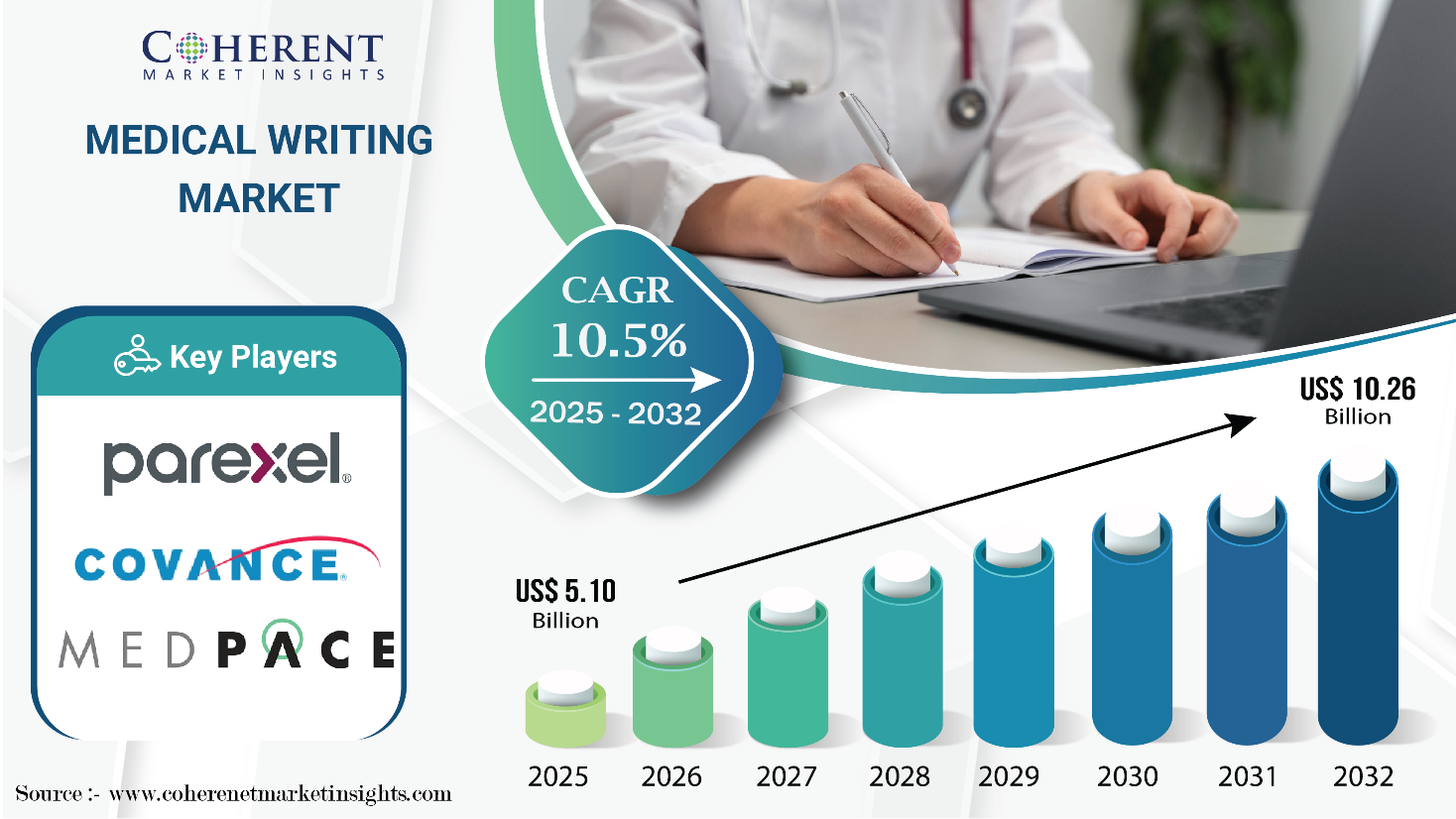
Burlingame, CA, July 01, 2025 (GLOBE NEWSWIRE) -- The Global Medical Writing Market is estimated to be valued at USD 5.10 Bn in 2025 and is expected to reach USD 10.26 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.5% from 2025 to 2032. This substantial growth can be largely attributed to the rising demand for regulatory documentation, clinical trial reports, and scientific publications within the pharmaceutical and healthcare sectors. This surge is partly fueled by the widespread adoption of clinical trial management systems like Medidata Solutions and Oracle Health Sciences. The medical writing market is experiencing a notable rise in outsourcing, as pharmaceutical and biotechnology companies aim to cut costs and enhance efficiency. Furthermore, the growing complexity of regulatory demands and the need for specialized knowledge are fueling the increasing demand for expert medical writing services.
Request Sample Report: https://www.coherentmarketinsights.com/insight/request-sample/8038
Global Medical Writing Market Key Takeaways
The global medical writing market size is projected to total USD 5.10 Bn in 2025, and further expand at a CAGR of 10.5% during the assessment period, reaching USD 10.26 Bn by 2032.
Based on type, clinical writing segment is anticipated to generate a market revenue of about USD 1.81 Bn in 2025.
In terms of application, oncology segment is projected to account for nearly one-fourth of the global medical writing market share in 2025.
By service provider, in-house medical writer category will likely hold a prominent market share of 54.3% by 2025.
As per CMI’s new medical writing market analysis, North America is expected to account for nearly two-fifths of the global medical writing market revenue share in 2025.
Asia Pacific medical writing market is poised to record fastest growth throughout the projection period.
Strict Regulatory Requirements Fueling Market Growth
Coherent Market Insights’ latest medical writing market report outlines major factors driving market growth. One such key growth driver is the increasingly stringent regulatory requirements imposed by health authorities.
Regulatory bodies like the FDA and EMA require well-documented and compliant submissions. This is driving demand for medical writing services as pharmaceutical and biotechnology companies look to comply with these regulations.
Pharmaceutical and biotech companies are constantly partnering with specialized medical writing services to ensure adherence to evolving regulatory frameworks. This trend is significantly boosting demand for expert medical writers who align scientific data with regulatory needs, thereby fueling market growth.
Data Privacy and High Cost of Services Restraining Market Growth
The prospective medical writing market outlook looks bright. However, high costs associated with professional medical writing services and persistent concerns over data privacy pose a barrier to widespread market expansion.
Outsourcing medical writing to professional agencies can be costly, especially for small and emerging biotech or pharmaceutical companies. This cost factor may negatively impact the overall medical writing market demand during the forecast period.
Many companies are also hesitant to outsource medical writing services due to the risk of data breaches. This can also limit medical writing market growth to some extent.
Buy This Premium Research Report at 25% Discount: https://www.coherentmarketinsights.com/insight/buy-now/8038
Booming Pharmaceutical and Biotech Industries Creating Growth Opportunities
Rapid expansion of pharmaceutical and biotechnology industries is expected to create lucrative growth prospects for medical writing companies. As these sectors expand, there is an increasing need for well-structured clinical documentation, regulatory submissions, and scientific publications.
Increasing drug development activities, clinical trials, and evolving regulatory requirements necessitate specialized medical writing expertise. Medical writers play a crucial role in translating complex scientific data into clear, compliant, and accessible content, thereby supporting product approvals and market launches.
Impact of AI on the Medical Writing Market
Artificial Intelligence (AI) is making strides in the medical writing market. It is helping companies streamline content creation as well as improve accuracy and accelerate turnaround times.
AI tools help with data analysis, literature reviews, and drafting regulatory or scientific documents. They reduce manual work, improve consistency, and ensure compliance. This lets medical writers focus on strategic, high-level tasks.
Many companies are following this trend by introducing AI-based solutions for medical writing. For instance, in June 2024, TrialAssure launched the new TrialAssure Link AI 2.0 medical writing technology. This updated version is designed to help pharma firms, CROs, biotech companies, and other service providers draft regulatory and medical documents using AI.
Similarly, in June 2024, Certara introduced its next-generation CoAuthor regulatory writing software. This advanced writing platform combines generative AI, Microsoft Word integration, document templates, and structured content authoring tools. It is specifically designed for medical writers.
Emerging Medical Writing Market Trends
Growing outsourcing trend is positively impacting the medical writing market value. Pharmaceutical and biotech companies are increasingly outsourcing medical writing tasks to specialized medical writing agencies and CROs to reduce costs, access skilled professionals, and focus more on their core R&D activities.
Rise in clinical trials worldwide is creating need for clinical trial protocols as well as investigator brochures and trial reports. This, in turn, will likely unlock growth opportunities for medical writing service providers.
Rapid advancements in fields like oncology, gene therapy, and rare diseases require specialized writing expertise to decode complex scientific information. This growing complexity is expected to boost demand for medical writing services in the coming years.
The healthcare industry’s shift towards digital transformation is creating new opportunities for medical writing service providers. There is a growing demand for content tailored to digital platforms, including telemedicine services, mobile health applications, and patient-facing digital tools. This trend is expanding the scope of medical writing beyond traditional regulatory and scientific documents.
Get Customization on this Report: https://www.coherentmarketinsights.com/insight/request-customization/8038
Analyst’s View
“The global medical writing industry is poised for significant growth, owing to expanding pharmaceutical and biotech sectors, rising demand for clinical trial documentation, and growing trend of outsourcing medical writing services,” said Komal Dighe, a senior analyst at CMI.
Current Events and Their Impact on the Medical Writing Market
| Event | Description and Impact |
| Widespread Adoption of Generative AI by CROs and Pharma Companies |
|
| Increased Use of Decentralized Clinical Trials (DCTs) |
|
| Surge in Orphan Drug and Cell/Gene Therapy Approvals |
|
Competitor Insights
Key companies listed in the medical writing market research report:
- Covance Inc.
- ICON plc
- Medpace Holdings, Inc.
- Parexel International Corporation
- Charles River Laboratories
- WuXi AppTec
- Syneos Health
- BioClinica
- PRA Health Sciences
- PPD (Pharmaceutical Product Development)
- KCR S.A.
- Celerion
- InVentiv Health
- FSP (Functional Service Provider
- Quorum Review IRB
Key Developments
In January 2025, AINGENS unveiled the Medical Affairs Content Generator (MACg), a novel multimodal, AI-powered medical writing and research assistant. This new solution is designed to revolutionize scientific content creation workflows in the life sciences sector.
In May 2025, Celerion launched the updated version of Labnotes, its bioanalytical data management software. This enhanced version is designed to elevate data handling and analysis capabilities as well as streamline laboratory documentation and improve operational efficiency for users.
Market Segmentation
Type Insights (Revenue, USD Bn, 2020 - 2032)
Clinical Writing
Regulatory Writing
Medical Education Writing
Medical Marketing Writing
Scientific Publications Writing
Application Insights (Revenue, USD Bn, 2020 - 2032)
Oncology
Cardiovascular
Neurology
Infectious Diseases
Rare Diseases
Others
Service Provider Insights (Revenue, USD Bn, 2020 - 2032)
In-house Medical Writers
Outsourced Medical Writing Services
End User Insights (Revenue, USD Bn, 2020 - 2032)
Pharmaceutical & Biotechnology Companies
Contract Research Organizations (CROs)
Medical Device Manufacturers
Healthcare Professionals
Academic & Research Organizations
Regulatory Authorities
Regional Insights (Revenue, USD Bn, 2020 - 2032)
North America
U.S.
Canada
Latin America
Brazil
Argentina
Mexico
Rest of Latin America
Europe
Germany
U.K.
Spain
France
Italy
Russia
Rest of Europe
Asia Pacific
China
India
Japan
Australia
South Korea
ASEAN
Rest of Asia Pacific
Middle East
GCC Countries
Israel
Rest of Middle East
Africa
South Africa
North Africa
Central Africa
Read Related Reports:
Medical Billing Outsourcing Market Size, Share & Trend Analysis Report (2025-2032)
Medical Affairs Outsourcing Market Size, Share, Trends & Opportunities for 2025-2032
Medical Claims Processing Services Market Size, Share Demand & Trend Analysis Report (2025-2032)
Our Trusted Partners:
Worldwide Market Reports, Coherent MI, Stratagem Market Insights
Get Recent News:
https://www.coherentmarketinsights.com/news

About Us: Coherent Market Insights leads into data and analytics, audience measurement, consumer behaviors, and market trend analysis. From shorter dispatch to in-depth insights, CMI has exceled in offering research, analytics, and consumer-focused shifts for nearly a decade. With cutting-edge syndicated tools and custom-made research services, we empower businesses to move in the direction of growth. We are multifunctional in our work scope and have 450+ seasoned consultants, analysts, and researchers across 26+ industries spread out in 32+ countries. Contact Us: Mr. Shah Coherent Market Insights Pvt. Ltd, U.S.: + 12524771362 U.K.: +442039578553 AUS: +61-2-4786-0457 INDIA: +91-848-285-0837 Email: sales@coherentmarketinsights.com For Latest Update Follow Us: LinkedIn | Facebook | Twitter
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
